Leaders
Task 1
To set-up randomized controlled trials of immunosuppressive biologic drugs in immune related adverse events post immune check-points
Samuel Bitoun is the French PI of a multicentric European prospective trial called CITAR.
This innovative randomized controlled clinical trial compares for the first time corticosteroids and tocilizumab (anti- IL-6R) in rheumatologic Immune-Related Adverse Events induced by ICI. Arianne Laparra (Gustave Roussy) participates by referring patients. CITAR is coordinated by Karolinska Institutet and 5 French centers participated in the CITAR clinical trial.

Watch the presentation of the CITAR Clinical Trial led by Samuel Bitoun, scientific coordinator FHU CARE²:
Task 2
To encourage randomized controlled trials with association of immunosuppressive biologic drugs and immune checkpoint in cancer to improve anti-cancer efficacy
FHU CARE² favor opening centers among members of the project in international trials testing the anticancer efficacy of adding immunomodulators to Immune Checkpoint Inhibitors. Recent evidence has shown the efficacy of combining JAKi and Immune Checkpoint Inhibitors in lung cancer (Mathew et al Science 2024) and Hodgkin’s lymphoma (Zaket al Science 2024).

A trial adding sarilumab (IL-6R inhibitor) to Immune Checkpoint Inhibitors in melanoma is actively recruiting (NCT05428007). Caroline Robert is a candidate for this trial promoted by the MD Anderson Cancer center (Texas US).


Task 3
To evaluate new treatment strategies based on innovative B-cell targeted therapies (BTK inhibitors, new generation anti-CD20) in lymphomas complicating autoimmune diseases
Gaëtane Nocturne is a leading expert on the field of Sjögren-associated lymphoma and initiates trials to test new drugs in lymphomas complicating autoimmune diseases and to assess their impact on Sjögren’s disease activity. Gaëtane Nocturne recently demonstrated that a systemic treatment strategy for Sjögren’s disease-associated lymphoma, rather than local treatment or watch and wait strategy, reduces the new Sjögren’s disease systemic activity risk (Rocca et al, Lancet Rheumatol 2024). Futures studies will evaluate the effect of innovative B-cell targeted therapies on lymphoma risk and assess the effect of new lymphoma treatment modalities on autoimmune diseases activity.
Task 4
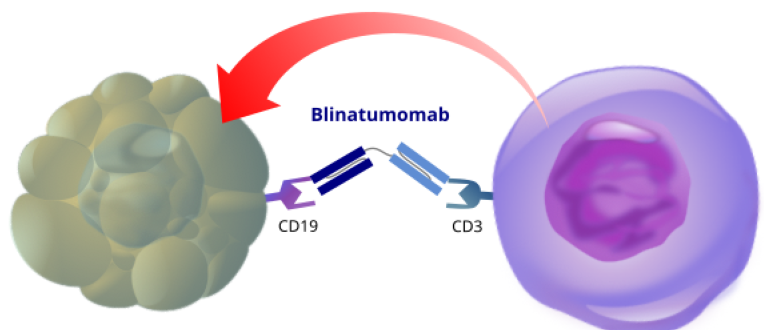
To develop in collaboration with industry master protocols for evaluating anti-CD19 CAR-T cells, anti-CD3/CD19 bispecific antibodies, anti-CD19 antibodies in patients with autoimmune diseases
Xavier Mariette with others recently created a multidisciplinary national group called Innovative Immunomodulatory drugs in IMIDs (Club C3i), on the umbrella of the national network on rare systemic autoimmune diseases (FAI2R). This group aims favoring all initiatives for developing innovative immunotherapies in autoimmune diseases and especially discussions with industry partners for developing master basket trials for evaluating their new drugs in patients with severe autoimmune diseases.
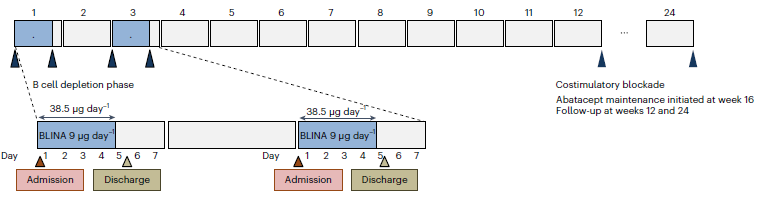

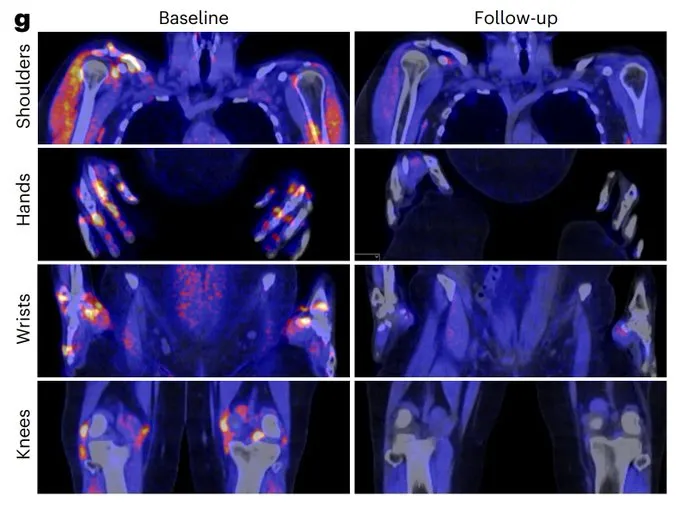
Task 5
To develop and preclinically test bispecific T-cell engager (BiTE) antibody and Chimeric autoantibody T-cells (CAAR-T cells) targeting the autoimmune B cells specifically.
With the expertise of Xavier Mariette, Samuel Bitoun, Raphaèle Seror and Gaëtane Nocturne (IDMIT UMR1184 /IMVA-HB) in Sjögren’s disease and the expertise of Bernard Maillere (CEA, Cellular Immunology and Biotechnology lab) in antibody engineering , new bispecific antibodies are being developped targetting CD3 and SSA. With the expertise of Camille Bigenwald and Stéphane de Botton (Hematology, Gustave Roussy) we will develop and produce SSA/ RF CAAR-T cells and test them in in vivo Sjögren disease models.

Jeanne Gauthier, PhD
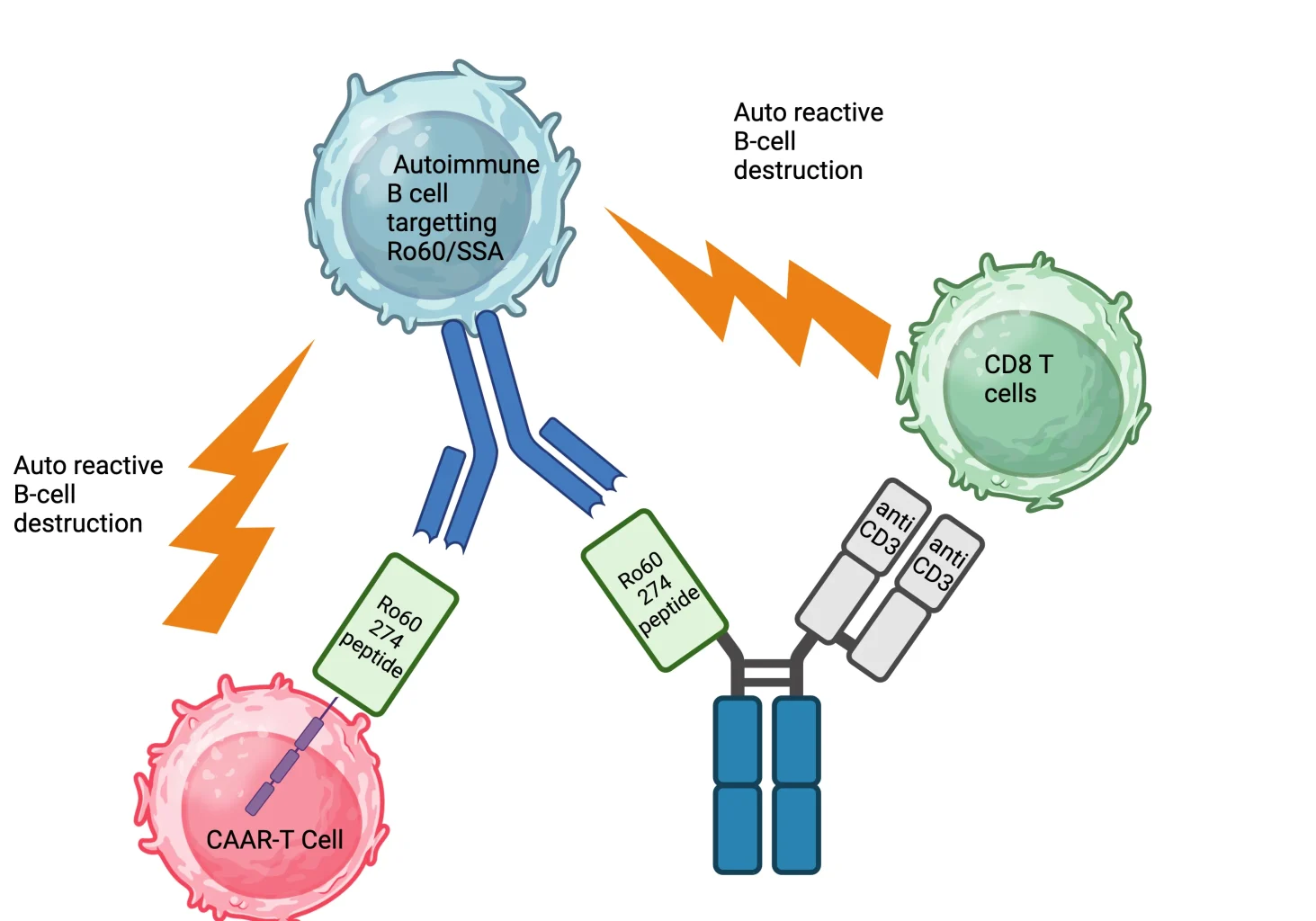
Task 6
To develop and preclinically test new CAR-T cell targets like CD30 and ROR1 in hematological and solid malignancies
A fully immunocompetent mouse model of CD19 CAR-T cells has been implemented by Camille Bigenwald (Hematology, Gustave Roussy). They demonstrated that the resistance to CD19 CAR-T cells is caused by defects in CAR-T cells occurring early or because of specific changes in the cancer immune environment, rather than by the loss of antigen expression or defects in CAR-T cell persistence. Therefore, using our relevant mouse model, combination therapies aimed at improving the effectiveness of CAR-T cells aretested with CD19 CAR-T cells but also with other settings such as CD30 CAR-T cells and ROR1 CAR-T cells. These approaches are interesting given the limited results in clinical trials for Hodgkin lymphoma and solid tumors.
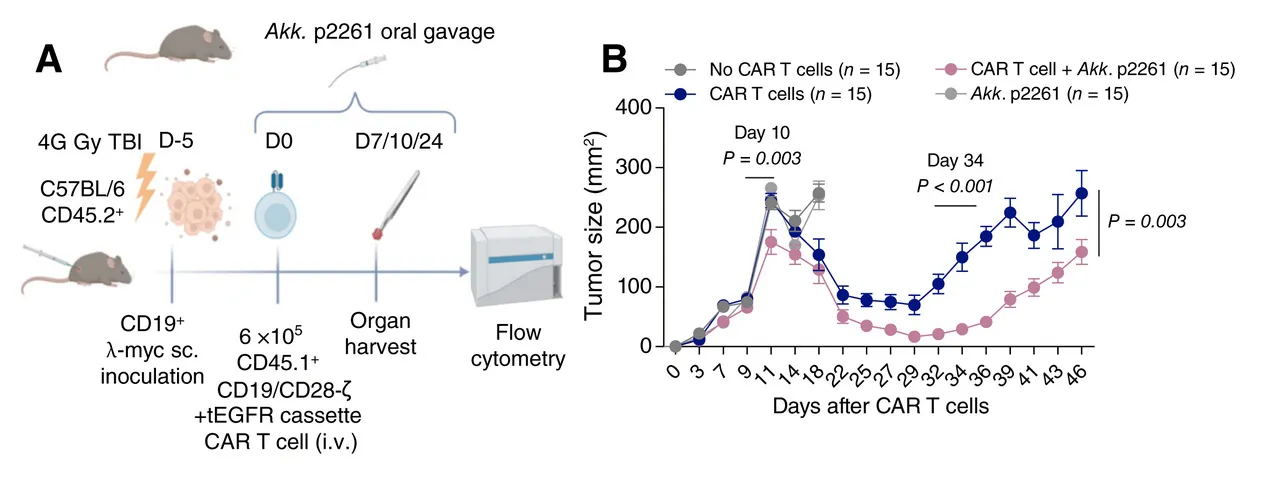
Task 7
To set-up a platform of intensive immunotherapy in autoimmune diseases and haematology in Bicêtre hospital and Institut Gustave Roussy. This allows to test CD19 CART-T cells and CAR-T cells
Camille Bigenwald has obtained from Institut Gustave Roussy to invest in the local production of human CAR-T and CAAR-T cells with the acquisition of a Miltenyi Prodigy machine.
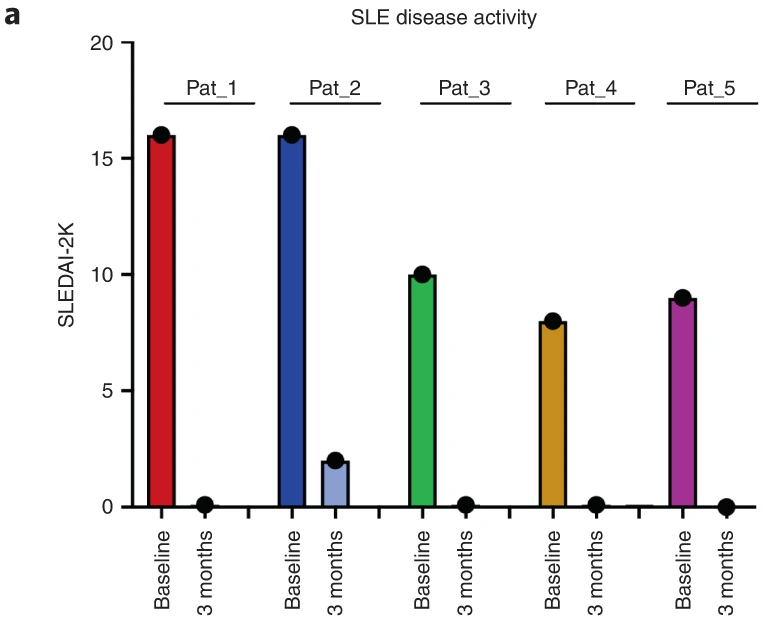
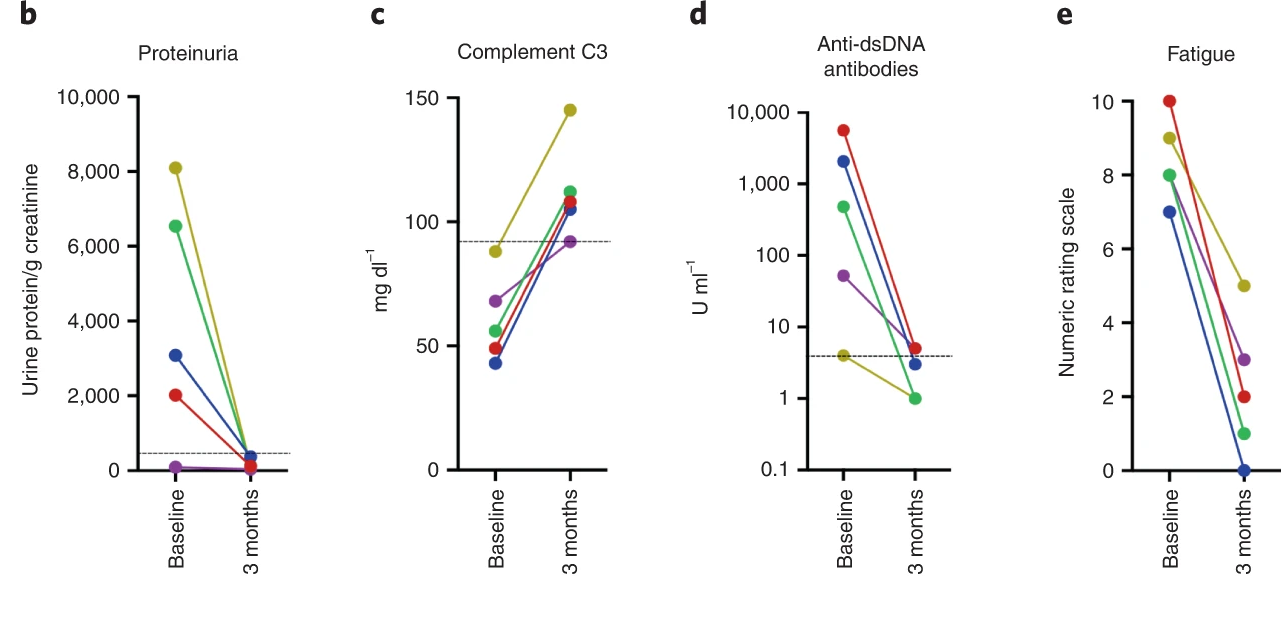
The machine was used by Müller et al (NEJM 2023) leading to the proof of concept of CD19 CAR-T cells efficacy in lupus patients.
This is allowed to produce CD19 CAR-T cells for unapproved indications like refractory autoimmune diseases (Samuel Bitoun and Xavier Mariette), and refractory pediatric lymphomas.
This paves the way for innovative CAR-T cell approaches such as CAAR-T and BiTEs.





